Abstract
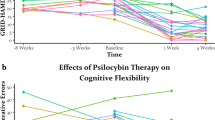
Psilocybin therapy shows antidepressant potential, but its therapeutic actions are not well understood. We assessed the subacute impact of psilocybin on brain function in two clinical trials of depression. The first was an open-label trial of orally administered psilocybin (10 mg and 25 mg, 7 d apart) in patients with treatment-resistant depression. Functional magnetic resonance imaging (fMRI) was recorded at baseline and 1 d after the 25-mg dose. Beck’s depression inventory was the primary outcome measure (MR/J00460X/1). The second trial was a double-blind phase II randomized controlled trial comparing psilocybin therapy with escitalopram. Patients with major depressive disorder received either 2 × 25 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily placebo (‘psilocybin arm’) or 2 × 1 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily escitalopram (10–20 mg) (‘escitalopram arm’). fMRI was recorded at baseline and 3 weeks after the second psilocybin dose (NCT03429075). In both trials, the antidepressant response to psilocybin was rapid, sustained and correlated with decreases in fMRI brain network modularity, implying that psilocybin’s antidepressant action may depend on a global increase in brain network integration. Network cartography analyses indicated that 5-HT2A receptor-rich higher-order functional networks became more functionally interconnected and flexible after psilocybin treatment. The antidepressant response to escitalopram was milder and no changes in brain network organization were observed. Consistent efficacy-related brain changes, correlating with robust antidepressant effects across two studies, suggest an antidepressant mechanism for psilocybin therapy: global increases in brain network integration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All requests for raw and analyzed data and materials are promptly reviewed by R.C.H. and D.J.N., chief investigator and principal investigator, respectively, on the original work. Patient-related data not included in the paper were generated as part of clinical trials and may be subject to patient confidentiality. Source data are provided with this paper.
Code availability
All analyses and data visualizations were conducted in MATLAB R2020a. Codes for generating each data figure are available at https://github.com/rdaws/psilodep.
References
Depression and Other Common Mental Disorders: Global Health Estimates (World Health Organization, 2017).
Tang, F. et al. COVID-19 related depression and anxiety among quarantined respondents. Psychol. Health 36, 164–178 (2021).
Rabeea, S. A., Merchant, H. A., Khan, M. U., Kow, C. S. & Hasan, S. S. Surging trends in prescriptions and costs of antidepressants in England amid COVID-19. DARU J. Pharm. Sci. https://doi.org/10.1007/s40199-021-00390-z (2021).
Hofmann, S. G., Curtiss, J., Carpenter, J. K. & Kind, S. Effect of treatments for depression on quality of life: a meta-analysis. Cogn. Behav. Ther. 46, 265–286 (2017).
Locher, C. et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry 74, 1011 (2017).
Haddad, P. The SSRI discontinuation syndrome. J. Psychopharmacol. 12, 305–313 (1998).
Steinert, C., Hofmann, M., Kruse, J. & Leichsenring, F. Relapse rates after psychotherapy for depression – stable long-term effects? A meta-analysis. J. Affect. Disord. 168, 107–118 (2014).
Nutt, D. & Carhart-Harris, R. The current status of psychedelics in psychiatry. JAMA Psychiatry https://doi.org/10.1001/jamapsychiatry.2020.2171 (2020).
Lyons, T. & Carhart-Harris, R. L. More realistic forecasting of future life events after psilocybin for treatment-resistant depression. Front. Psychol. 9, 1721 (2018).
Beck, A. T. & Clark, D. A. Anxiety and depression: an information processing perspective. Anxiety Res. 1, 23–36 (1988).
Rolls, E. T. A non-reward attractor theory of depression. Neurosci. Biobehav. Rev. 68, 47–58 (2016).
Hamilton, J. P. et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry 70, 327–333 (2011).
Kendler, K. S. The phenomenology of major depression and the representativeness and nature of DSM criteria. Am. J. Psychiatry 173, 771–780 (2016).
Goodman, Z. et al. Whole-brain functional dynamics track depressive symptom severity. Cereb. Cortex https://doi.org/10.1093/cercor/bhab047 (2021).
Margulies, D. S. et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016).
Andrews-Hanna, J. R., Smallwood, J. & Spreng, R. N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance: the brain’s default network. Ann. NY Acad. Sci. 1316, 29–52 (2014).
Lydon-Staley, D. M. et al. Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Transl. Psychiatry 9, 234 (2019).
Daws, R. E. et al. Optimisation of brain states and behavioural strategies when learning complex tasks. Preprint at bioRxiv https://doi.org/10.1101/2020.06.17.156570 (2021).
Kim, C., Cilles, S. E., Johnson, N. F. & Gold, B. T. Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum. Brain Mapp. 33, 130–142 (2012).
Turnbull, A. et al. Reductions in task-positive neural systems occur with the passage of time and are associated with changes in ongoing thought. Sci. Rep. 10, 9912 (2020).
Wilkinson, P. O. & Goodyer, I. M. Attention difficulties and mood-related ruminative response style in adolescents with unipolar depression. J. Child Psychol. Psychiatry https://doi.org/10.1111/j.1469-7610.2006.01660.x (2006).
Vollenweider, F. X., Vollenweider-Scherpenhuyzen, M. F. I., Bäbler, A., Vogel, H. & Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport 9, 3897–3902 (1998).
Beliveau, V. et al. The structure of the serotonin system: a PET imaging study. NeuroImage 205, 116240 (2020).
Andersen, K. A. A., Carhart‐Harris, R., Nutt, D. J. & Erritzoe, D. Therapeutic effects of classic serotonergic psychedelics: a systematic review of modern‐era clinical studies. Acta Psychiatr. Scand. 143, 101–118 (2021).
Carhart-Harris, R. L. et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 7, 13187 (2017).
Carhart-Harris, R. L. et al. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384, 1402–1411 (2021).
Nutt, D., Erritzoe, D. & Carhart-Harris, R. Psychedelic psychiatry’s brave new world. Cell 181, 24–28 (2020).
Carhart-Harris, R. L. & Friston, K. J. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol. Rev. 71, 316–344 (2019).
Lord, L.-D. et al. Dynamical exploration of the repertoire of brain networks at rest is modulated by psilocybin. NeuroImage 199, 127–142 (2019).
Luppi, A. I. et al. LSD alters dynamic integration and segregation in the human brain. NeuroImage 227, 117653 (2021).
Carhart-Harris, R. L. et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl Acad. Sci. USA 109, 2138–2143 (2012).
Fried, E. I. The 52 symptoms of major depression: lack of content overlap among seven common depression scales. J. Affect. Disord. 208, 191–197 (2017).
Mattar, M. G., Cole, M. W., Thompson-Schill, S. L. & Bassett, D. S. A functional cartography of cognitive systems. PLoS Comput. Biol. 11, e1004533 (2015).
Mucha, P. J., Richardson, T., Macon, K., Porter, M. A. & Onnela, J.-P. Community structure in time-dependent, multiscale, and multiplex networks. Science 328, 876–878 (2010).
Barrett, F. S., Doss, M. K., Sepeda, N. D., Pekar, J. J. & Griffiths, R. R. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 10, 2214 (2020).
Pasquini, L., Palhano-Fontes, F. & Araujo, D. B. Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J. Psychopharmacol. 34, 623–635 (2020).
Parkes, L., Satterthwaite, T. D. & Bassett, D. S. Towards precise resting-state fMRI biomarkers in psychiatry: synthesizing developments in transdiagnostic research, dimensional models of psychopathology, and normative neurodevelopment. Curr. Opin. Neurobiol. 65, 120–128 (2020).
Ye, M. et al. Changes of functional brain networks in major depressive disorder: a graph theoretical analysis of resting-state fMRI. PLoS ONE 10, e0133775 (2015).
Feurer, C. et al. Resting state functional connectivity correlates of rumination and worry in internalizing psychopathologies. Depress. Anxiety https://doi.org/10.1002/da.23142 (2021).
Roseman, L. et al. Emotional breakthrough and psychedelics: validation of the emotional breakthrough inventory. J. Psychopharmacol. 33, 1076–1087 (2019).
Watts, R., Day, C., Krzanowski, J., Nutt, D. & Carhart-Harris, R. Patients’ accounts of increased ‘connectedness’ and ‘acceptance’ after psilocybin for treatment-resistant depression. J. Humanist. Psychol. 57, 520–564 (2017).
Murphy-Beiner, A. & Soar, K. Ayahuasca’s ‘afterglow’: improved mindfulness and cognitive flexibility in ayahuasca drinkers. Psychopharmacol. 237, 1161–1169 (2020).
Zeifman, R. J. et al. Post-psychedelic reductions in experiential avoidance are associated with decreases in depression severity and suicidal ideation. Front. Psychiatry 11, 782 (2020).
Carhart-Harris, R. L. & Nutt, D. J. Serotonin and brain function: a tale of two receptors. J. Psychopharmacol. 31, 1091–1120 (2017).
Wang, X., Öngür, D., Auerbach, R. P. & Yao, S. Cognitive vulnerability to major depression: view from the intrinsic network and cross-network interactions. Harv. Rev. Psychiatry 24, 188–201 (2016).
Hampshire, A. et al. Probing cortical and sub-cortical contributions to instruction-based learning: regional specialisation and global network dynamics. NeuroImage 192, 88–100 (2019).
Soreq, E., Violante, I. R., Daws, R. E. & Hampshire, A. Neuroimaging evidence for a network sampling theory of individual differences in human intelligence test performance. Nat. Commun. 12, 2072 (2021).
Watanabe, T. & Rees, G. Brain network dynamics in high-functioning individuals with autism. Nat. Commun. 8, 16048 (2017).
Gu, B.-M. et al. Neural correlates of cognitive inflexibility during task-switching in obsessive–compulsive disorder. Brain 131, 155–164 (2007).
Wei, M. et al. Abnormal dynamic community structure of the salience network in depression: abnormal salience network in depression. J. Magn. Reson. Imaging 45, 1135–1143 (2017).
Carhart-Harris, R. L. et al. Can pragmatic research, real-world data and digital technologies aid the development of psychedelic medicine? J. Psychopharmacol. https://doi.org/10.1177/02698811211008567 (2021).
Carhart-Harris, R. L. et al. Psychedelics and the essential importance of context. J. Psychopharmacol. 32, 725–731 (2018).
Tagliazucchi, E. & Laufs, H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron 82, 695–708 (2014).
Leonardi, N. & Van De Ville, D. On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage 104, 430–436 (2015).
Finc, K. et al. Dynamic reconfiguration of functional brain networks during working memory training. Nat. Commun. 11, 2435 (2020).
Jack, C. R. et al. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27, 685–691 (2008).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. NeuroImage 9, 179–194 (1999).
Avants, B. B., Tustison, N. & Song, G. Advanced normalization tools (ANTs). Insight J. 2, 1–35 (2009).
Schaefer, A. et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex 28, 3095–3114 (2018).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
Newman, M. E. J. & Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 69, 026113 (2004).
Bassett, D. S. et al. Task-based core-periphery organization of human brain dynamics. PLoS Comput. Biol. 9, e1003171 (2013).
Maslov, S. & Sneppen, K. Specificity and stability in topology of protein networks. Science 296, 910–913 (2002).
Rubinov, M. & Sporns, O. Weight-conserving characterization of complex functional brain networks. NeuroImage 56, 2068–2079 (2011).
Acknowledgements
R.E.D. was supported by an Engineering and Physical Sciences Research Council PhD scholarship at the Imperial College London Centre for Neurotechnology (EP/L016737/1). The research was carried out at the National Institute for Health Research/Wellcome Trust Imperial Clinical Research Facility. The open-label trial was funded by a Medical Research Council clinical development scheme grant (MR/J00460X/1). The DB-RCT was funded by a private donation from the Alexander Mosley Charitable Trust, supplemented by Founders of Imperial College London’s Centre for Psychedelic Research.
Author information
Authors and Affiliations
Contributions
This study was designed and planned by R.C.-H. and D.N. and conducted by B.G., M.B.W., D.E. and L.R. The specific analysis was designed by R.E.D. and C.T. The analysis was conducted and visualized by R.E.D. The manuscript was drafted by R.E.D., C.T. and R.C.-H. All authors contributed to the interpretation of the study results and revised and approved the manuscript for intellectual content. The corresponding author (R.E.D.) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
R.C.H. reports receiving consulting fees from Entheon Biomedical and Beckley Psytech; B.G. received consulting fees from SmallPharma; D.E. received consulting fees from Field Trip and Mydecine; D.N. received consulting fees from Algernon and H. Lundbeck and Beckley Psytech, advisory board fees from COMPASS Pathways and lecture fees from Takeda and Otsuka and Janssen plus owns stock in Alcarelle, Awakn and Psyched Wellness. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks David Hellerstein, Jared Van Snellenberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Tables 1 and 2.
Supplementary Data 1
Data plotted in Supplementary Fig. 1.
Supplementary Data 2
Data plotted in Supplementary Fig. 2.
Supplementary Data 3
Data plotted in Supplementary Fig. 3.
Supplementary Data 4
Data plotted in Supplementary Fig. 4.
Source data
Source Data Fig. 3
BDI scores (one tab per trial).
Source Data Fig. 4
Modularity (Q) baseline and post-treatment, BDI and DMN, EN and SN integration scores (open-label trial).
Source Data Fig. 5
Modularity (Q) baseline and post-treatment, BDI and 7 × 7 network correlation coefficients and P values for each arm (DB-RCT).
Rights and permissions
About this article
Cite this article
Daws, R.E., Timmermann, C., Giribaldi, B. et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med 28, 844–851 (2022). https://doi.org/10.1038/s41591-022-01744-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-022-01744-z
This article is cited by
-
Convergent functional effects of antidepressants in major depressive disorder: a neuroimaging meta-analysis
Molecular Psychiatry (2025)
-
Whole-brain turbulent dynamics predict responsiveness to pharmacological treatment in major depressive disorder
Molecular Psychiatry (2025)
-
Analysis of deep non-smooth symmetric nonnegative matrix factorization on hierarchical clustering
Applied Intelligence (2025)
-
Multimodal creativity assessments following acute and sustained microdosing of lysergic acid diethylamide
Psychopharmacology (2025)
-
Engaging Mood Brain Circuits with Psilocybin (EMBRACE): a study protocol for a randomized, placebo-controlled and delayed-start, neuroimaging trial in depression
Trials (2024)